
San Carlos, CA — International Process Solutions , a leader in precision calibration and validation services, is proud to announce its comprehensive one-stop-shop approach for organizations in regulated industries. By offering integrated calibration, validation, and compliance solutions under a single provider, IPS simplifies operations, ensures audit-ready documentation, and enhances operational efficiency for businesses across pharmaceuticals, biotechnology,

Irvine, CA — International Process Solutions, a leading provider of calibration, validation, and compliance services across California and the United States, is proud to announce the expansion of its specialized Validation Services, offering advanced support for a broad range of equipment essential to regulated industries. With a focus on precision, reliability, and regulatory excellence, IPS

San Carlos, California – 9/18 – International Process Solutions (IPS), a leading provider of precision calibration and laboratory solutions, is proud to announce that it has achieved ISO 17025 accreditation for its pipette calibration services. This milestone reflects IPS’s unwavering commitment to quality, accuracy, and reliability in laboratory instrumentation, further solidifying its position as an
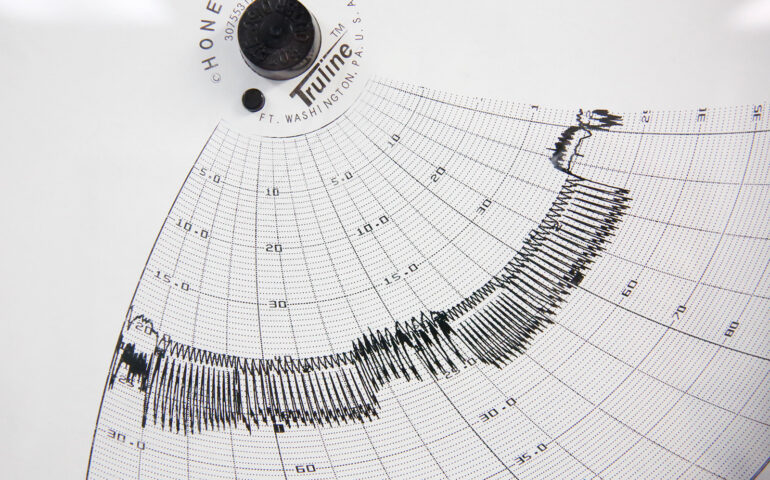
Irvine, CA – International Process Solutions (IPS), a leading provider of calibration, validation, and environmental monitoring services, is proud to announce the launch of its on-site calibration services with guaranteed quick turnaround times of up to 72 hours. This new service is designed to meet the urgent demands of industries where precision and compliance are

San Carlos, CA – July 29, 2025 – International Process Solutions (IPS), a leading provider of calibration and validation services for the life sciences industry, today announced the expansion of its pipette calibration services across the state of California. This growth initiative comes as a direct response to the rapidly increasing demand from biotechnology, pharmaceutical,

April 2025 — International Process Solutions (IPS), a premier provider of precision calibration and validation services, is proud to announce the expansion of its offerings to include Ultrasonic Flow Calibration services across both Southern and Northern California. This new service is designed to meet the increasing demand for highly accurate flow measurement in industries such

San Diego, CA – International Process Solutions (IPS), a leading provider of calibration and validation services, is proud to announce the expansion of its comprehensive suite of offerings, making it a true one-stop shop for businesses in highly regulated industries. With an unwavering commitment to quality, accuracy, and compliance, IPS now provides fully integrated calibration

San Carlos 1/15/2025 – International Process Solutions (IPS) is a leading provider of calibration services in California, specializing in the pharmaceutical and biotech industries. Their services are designed to meet cGMP (current Good Manufacturing Practices) standards, ensuring regulatory compliance and precision for their clients. Calibration Services Offered: GMP Calibration Regulatory Maintenance: IPS focuses on cGMPs,

San Carlos, CA – International Process Solutions (IPS), a leading provider of precision calibration and validation services, continues to set the industry standard by offering a comprehensive suite of solutions tailored to meet the diverse needs of the pharmaceutical, bioprocess, and general laboratory sectors. With a commitment to precision, reliability, and compliance, IPS ensures that
San Carlos, California – October 5, 2024 — International Process Solutions (IPS), a leading provider of ISO-accredited calibration services, is excited to announce the expansion of its calibration offerings with the introduction of spectrophotometer calibration services in Northern California. This new service aims to support laboratories and industries that rely on precise color measurement and
Recent Comments